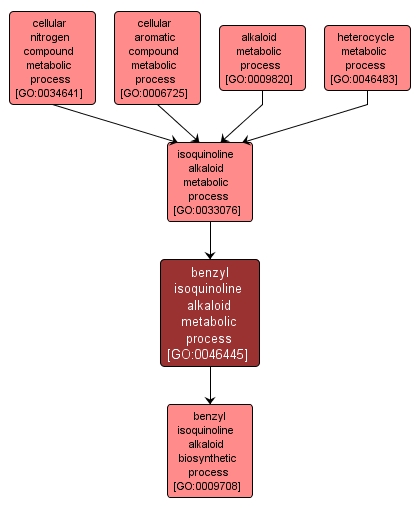
GO TERM SUMMARY
|
| Name: |
benzyl isoquinoline alkaloid metabolic process |
| Acc: |
GO:0046445 |
| Aspect: |
Biological Process |
| Desc: |
The chemical reactions and pathways involving benzyl isoquinoline alkaloids, compounds with bicyclic N-containing aromatic rings. |
Synonyms:
- benzyl isoquinoline alkaloid metabolism
|
|

|
INTERACTIVE GO GRAPH
|